Chemical Reactions and Equations Blog 4
Dear Learners,
I hope you all are following the instruction given in previous blogs.
I also presume that you all are well aware of writing and balancing of chemical
equations.
I feel you all have not only taken the notes but also completed
the home assignment in your respective chemistry note book.
In the previous blog, we have discussed writing and balancing
chemical equation. I must help you to recall
(i) Chemical reactions are balanced to follow law of conservation
of mass.
(ii) Follow the rules to balance the chemical equation.
Now let us discuss the very important topic- TYPES OF CHEMICAL
REACTIONS
Types of reaction-
Combination, Decomposition and Single Displacement reactions will be discussed
in todays blog.
Happy Learning!
At the end of this topic, learners will be able to
(i) define combination reactions, decomposition reactions,
displacement reactions.
(ii) write the reactions of above stated chemical reactions.
(iii) apply the reactions in your real life.
(iv) give correct answer based on activities given in your text
book.
(iv) write the correct answer in your board exams.
Key Instructions:
Green lines are meant to be
written in note book.
Home Assignment need to be done in note book.
So, Let us begin our topic TYPE OF CHEMICAL REACTION
As we all aware that during chemical reaction atoms of one element
do not change into those of another element. Nor do atoms disappear from the
mixture or appear from elsewhere. Actually, chemical reactions involves the
breaking and making of bonds between atoms to produce new substances.
NOTE: You all will study about types of bonds formed between atoms
in Chapter 3 and 4.
Let us study here first types of chemical reactions.
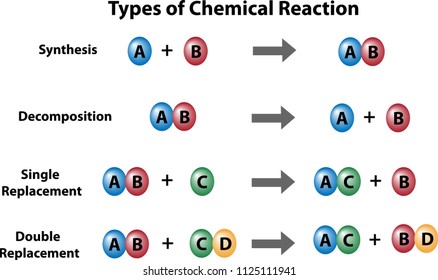

The first reaction is
1. Combination Reaction: When
two or more substances (element or compounds) combine to form a single product,
the reactions are called combination reactions.
Example of Combination Reaction
happening between two elements
(ii) Formation of water from H2 and O2 2H2 + O2 à 2H2O
Example of Combination Reaction
happening between two compounds
Refer Activity 1.4 NCERT Page 6 Chapter 1
(i) Reaction between Quick Lime
(CaO) and water
In this reaction, a single product is formed from two or more
reactant is known as a combination reactions. Dear learners, you should note
here that large amount of heat is also released in this reaction which will be
discussed in next topic.
Now let us take few more examples of combination reactions
Example 1: Hydrogen combines with chlorine to form hydrogen
chloride.H2 + Cl2 à 2HCl
Example 2: Sodium metal burns in chlorine to form sodium chloride.
2Na + Cl2 à 2NaCl
Example 3: iron is heated with Sulphur, it forms iron sulphide.
Fe + S à FeS
Example 4: Ammonia reacts with hydrogen chloride to form ammonium chloride.
NH3 + HCl à NH4Cl
Example 5: Carbon monoxide reacts with oxygen to form carbon dioxide.
2CO + O2 à 2CO2
Example 6: Sulphur dioxide reacts with oxygen to produce Sulphur trioxide.
2SO2 + O2 à 2SO3
I hope you all are now well aware of combination reactions. So
now, let us move to next reaction.
https://www.youtube.com/watch?v=-0DCopjuCwg2. Decomposition Reaction
Those reaction in which a compound splits up into two or more simpler substances are known as decomposition. The decomposition reactions are carried out by applying heat, light or electricity which provide energy to break a compound into two or more simpler compounds. When a decomposition reaction is carried out by heating, it is called ‘thermal decomposition reaction’. When a decomposition reaction is carried out by passing electricity, it is called ‘electrical decomposition reaction. Decomposition reaction can also be carried by light energy.
Thermal Decomposition:
Example 1: when calcium carbonate is heated, it decomposes to give calcium oxide and carbon dioxide.
CaCO3 à CaO + CO2
Decomposition of calcium carbonate to calcium oxide and carbon dioxide on heating is thermal decomposition. Calcium oxide (CaO) is called lime or quick lime. It has used in manufacture of cement.
Example 2: when potassium chlorate is heated in the presence of manganese dioxide catalyst, it decomposes to give potassium chloride and oxygen.
2KClO3 à 2KCl + 2O2
Example 3: Refer activity 1.5 when ferrous sulphate is heated strongly, it decomposes to form ferric oxide, Sulphur dioxide and Sulphur trioxide.
2FeSO4 à Fe2O3 + SO2 + SO3
Example 4:Refer activity 1.6 when lead nitrate is heated strongly, it breaks down to forms lead monoxide, nitrogen dioxide and oxygen.
2Pb(NO3)2 à 2PbO + 4NO2 + O2
Electrical Decomposition
Example 1: refer
activity 1.7 when electric current is passed through acidified water, it
decomposes to give hydrogen gas and oxygen gas. 2H2O à 2H2 + O2
2NaCl à 2Na
+ Cl2
Example 3: when
electric current is passed through molten aluminium oxide, it decompose to give
aluminium metal and oxygen. 2Al2O3 à 4Al + 3O2
Example 1: Refer activity 1.8 when silver chloride is exposed to light, it decomposes to form silver metal and chlorine gas.
2AgCl à 2Ag + Cl2
Example 2: Silver bromide decompose into silver and bromine gas.
2AgBr à 2Ag + Br2
Uses of Decomposition Reaction:
The decomposition reaction carried out by electricity are used to extract several metals form their naturally occurring compounds like chlorides or oxides. When the fused molten metal chloride or metal oxide is decomposed by passing electricity, then metal is produced at the cathode (negative electrode). To be discussed in detail in chapter 3.
Decomposition Reaction in our body: the digestion of food in the body is decomposition reaction. When we eat food which contains starch it decompose into simple sugar, protein decomposes into amino acids.
3. Displacement Reaction:
Refer Activity 1.9 Page 10 Chapter 1 NCERT
In the reaction, iron nail become brownish in colour and the blue colour of copper sulphate fades. The following chemical reaction takes place in this activity-
Fe + CuSO4 à FeSO4 + Cu
In this reaction, iron has displaced or removed another element, copper, from copper sulphate solution. This reaction is known as displacement reaction.
Other examples of displacement reactions are
Example 1: zinc metal is placed in copper sulphate solution, then zinc sulphate solution and copper are obtained.
Zn + CuSO4 à ZnSO4 + Cu
Example 3: Lead metal is placed in a solution of copper chloride then lead chloride and copper metal is formed.
CuCl2 + Pb à PbCl2 + Cu
Zinc and lead are more reactive elements than copper. They displace copper from its compounds.
Example 4: Copper reacts with silver nitrate then copper nitrate and silver metal is formed.
2AgNO3 + Cu à Cu(NO3)2 + 2Ag
Example 2: Magnesium metal is placed in copper sulphate solution then magnesium sulphate solution and copper metal is formed.
Mg + CuSO4 à MgSO4 + Cu
https://www.youtube.com/watch?v=r2u3J0dDsfU
Home Assignments
1. Complete your notebook.
2. A
colourless lead salt, when heated, produces a yellow residue and brown fumes.
i. Name the lead salt.
ii. Name the brown fumes.
3. What
happens when silver chloride is exposed to sunlight? Write a chemical equation
for this reaction. Also give one use of such a reaction.
4. Why
are decomposition reactions called the opposite of combination reactions?
Explain with equations of these reactions.
5. When
a green iron salt is heated strongly, its colour finally changes to black and
odour of burning Sulphur is given out.
a) Name the iron salt.
b)
Name the type of reaction that takes place
during the heating of iron salt.
c) Write
a chemical equation of the reaction involved.

Good Morning Sir
ReplyDelete-Ojas Girotra
Good morning Sir
ReplyDeleteAdavya Dhir X-D
10D
ReplyDeleteGood morning sir
ReplyDeleteHarsh vardhan
Good morning sir
ReplyDeleteEmmanuel gomes
Good morning sir
ReplyDeleteSwyam Kapoor
good morning sir
ReplyDeleteEugene Samuel
Good morning sir
ReplyDeleteKennard Rence William
Good morning sir
ReplyDeleteAkshat Chadha
Dear all,
ReplyDeleteIn chemical reactions, a' is to be replaced by arrow key.
Refer ncert.
Good morning sir
ReplyDeleteAkshat Verma
Good Morning Sir
ReplyDelete-Pratham Chhabra
Good morning sir
ReplyDeleteGood Morning sir
ReplyDeleteShaurya Narang
Good morning sir
ReplyDeleteKshitij Jain
Good morning sir
ReplyDeleteSidh chichra
PRESENT
ReplyDeleteGood morning sir.
ReplyDeleteDivij Gogia
Good morning sir
ReplyDeleteSharon Santhosh X-D
Good morning sir
ReplyDeleteBennett Joy
good morning sir
ReplyDeleteGOOD MORNING SIR
ReplyDeleteANSHUMAAN
Good morning sir
ReplyDeleteGood morning sir
ReplyDeleteJapmann singh
good morninig sir
ReplyDeleterudraksh
Good morning Ma'am
ReplyDeleteSarim Hasan
Good morning sir
ReplyDeleteShaurya Shil
Good morning sir Arvind Goyal
ReplyDeleteGood morning sir
ReplyDeleteSahej Grover
10-D
Good Morning Sir
ReplyDeleteAnmol Singh